At the Needle Point: The Role of Materials in a Coronavirus Vaccine
The development of a vaccine for the novel coronavirus SARS-CoV-2 has captured—perhaps uniquely—the attention of a global audience of scientists, medical doctors, politicians, and the general public. However, identifying a viable vaccine candidate is only one of the first, of many, steps in eradicating the dangers of COVID-19, the disease caused by the novel coronavirus. Rapid production scale-up, which is already initiated, and the enormous logistical hurdle of distributing the vaccine to the entire population of the world are still in front of us. Such a large quantity of vaccine requires an equally large amount of specialized materials to bottle and deliver the drug. In fact, despite assurances from medical device makers, several drug makers have raised concerns that there may be a lack of enough specialized glass vials to conduct an immunization campaign on a global scale.
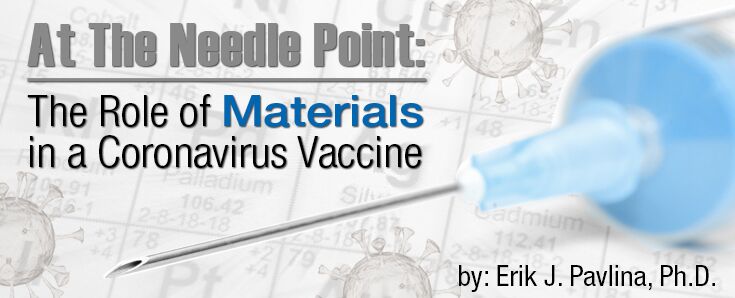
Modern drug delivery systems are comprised of a variety of materials—glass, polymers, ceramics, and metals. For many of us, our interaction with vaccines is at the point of a needle. Early hypodermic needles of the 19th century were made from silver. Today, most hypodermic needles are made from an austenitic stainless steel, such as Type 304L stainless steel—the same stainless steel commonly used in kitchen sinks. Austenitic stainless steels find widespread use in medical devices as a result of a good combination of biocompatibility, corrosion resistance, strength, manufacturability, and cost. These attributes are necessary to produce hollow hypodermic needles to incredibly fine sizes through a repeated process of tube drawing and annealing (heating to a high temperature in order to soften the steel). The finest needles have an outside diameter of about two tenths of a millimeter—approximately twice the diameter of a human hair—with inner diameters about half as much!
Steels, by definition, are iron-based alloys containing less than approximately 2% carbon, by weight. Stainless steels achieve their corrosion resistance by the addition of large amounts of chromium—more than 11% by weight—to steel compared to 1% or less in typical structural steels. The chromium in the stainless steel spontaneously reacts with oxygen in the air to form a thin, stable, nonporous chromium oxide layer on the outer surface of the steel. It is this chromium oxide layer that provides protection to the steel in corrosive environments and protects the patient against adverse toxicological or immunological responses resulting from contact with the metal. The chromium oxide layer forms without any intervention—other than exposing the steel to air—by the manufacturer, fabricator, or user.
During the steelmaking process, other oxides such as iron oxide—which offer little corrosion protection—form on the surface of the stainless steel. Damage resulting from the manufacturing process can also disturb the beneficial chromium oxide surface layer and introduce other contaminants that promote corrosion. In these situations, many manufacturers will subject the stainless steel to a passivation treatment as a final step prior to use. Passivation intentionally removes any iron or iron-based compounds present on the surface of the steel in order to restore an uniform, protective chromium oxide layer. Passivation involves passing the steel through a bath of nitric acid or citric acid followed by a neutralizing agent. The steel is then usually tested to determine the efficacy of the passivation treatment.
Despite being passivated, stainless steels can still corrode under certain conditions or after common processes. Attack by chlorides in water at moderate temperatures (65 °C or 150 °F) is a common form of corrosive attack of austenitic stainless steel. The addition of molybdenum to the stainless steel—such as Type 316 stainless steel—improves the resistance to chloride attack.
Sensitization occurs when the stainless steel is heated to elevated temperatures at which chromium reacts with carbon in the steel to form chromium carbide. The formation of chromium carbide depletes the local concentration of chromium at internal grain boundaries within the steel; corrosive attack can then occur at these local areas of chromium depletion. Sensitization is often associated with welding, which introduces high heat input followed by relatively slow cooling rates—both of which are favorable for the formation of chromium carbide. Limits to the amount of free carbon in the steel as well as post weld heat treatments are often employed to combat sensitization.
Sterilization—common in the medical device community—by gamma irradiation has been shown to reduce the corrosion resistance of some stainless steels. The reduced corrosion resistance results from a localized breakdown of the chromium oxide layer as a result of the irradiation. Autoclaving—a sterilization technique using pressurized steam—has been shown to improve corrosion resistance as a result of increasing the thickness of the chromium oxide layer.
Ultimately, success in the global fight against the novel coronavirus will be determined by pairing the appropriate drugs, materials, and supply chain resources that are being developed and delivered by dedicated scientists, medical personnel, engineers, analysts, and more. Even though the forthcoming months are filled with uncertainty for many of us, early clinical vaccine trial results show promise on multiple fronts. We should also take comfort that we have safe, reliable materials to meet this challenge, because new developments in medical technology cannot occur without advanced materials.

Erik J. Pavlina, Ph.D. – Associate II, Cincinnati Office
Dr. Erik Pavlina specializes in processing and manufacturing of metals, materials selection, and failure analysis. He brings extensive and broad experience in manufacturing, industrial R&D and product development, and fundamental research in ferrous and non-ferrous alloys to his role at Stress Engineering Services. Dr. Pavlina completed his MS and PhD at Colorado School of Mines focusing on ferrous metals.




Leave a Comment
You must Register or Login to post a comment.